Effective aluminum in zinc liquid
The effective aluminum in the zinc liquid means the amount of aluminum that has been removed from the total aluminum and has become an aluminum zinc compound and is dissolved in the zinc liquid in a free state and reacts to the galvanizing process.
The method for determining the content of effective aluminum in zinc liquid is: first analyze the total aluminum content and total iron content in the zinc liquid first, then calculate the content of Fe2Al5 in the zinc residue according to the total iron content, and further calculate the aluminum content in Fe2Al5. The content, in order to remove this amount from the total amount of aluminum, results in an effective aluminum content.
In terms of theoretical analysis, the calculation of effective aluminum is very complicated. Several assumptions can be made in the actual production, that is, regardless of the solubility of aluminum in zinc liquid with temperature, 0.03% of conventional data is taken, and the aluminum in the zinc pot All exceed 0.135%, the iron and zinc reaction compounds are Fe2Al5Znx, the effective aluminum formula is x effective Al = x total A1-(xFe-0.03%) × 0.85
In the formula, x effective Al is the content of effective aluminum in zinc liquid, %; x total A1 is the total aluminum content in zinc liquid, %; xFe is the iron content in zinc liquid, %.
At present, foreign research on effective aluminum is more in-depth. A measurement probe and software specially designed for measuring the effective aluminum content in zinc liquid have been researched and developed. The system can simultaneously detect the aluminum content and iron content in zinc liquid and measure it. The temperature of the zinc liquid, taking into account the changes in the solubility of iron at different temperatures, can accurately calculate the effective aluminum content, and determine whether the main components of the zinc slag is FeZnl3, FeZn, or Fe2A12Znx.
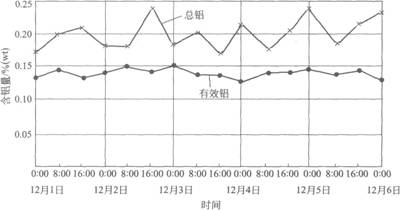
Effective aluminum and total aluminum curve in zinc pot
A set of valid aluminum and total aluminum data curves for a foreign company. It can be seen from the figure that the total amount of aluminum in the zinc liquid varies greatly with the amount of zinc dross, that is, the change of the sampling position, while the fluctuation of the effective aluminum is very small, which can better reflect the aluminum in the zinc liquid. Impact on the product. Therefore, controlling the total amount of aluminum in the zinc liquid is only a relatively primitive, crude management method that is likely to be misleading and most likely reflects the actual amount of available aluminum.
According to theoretical analysis and practical verification, the recommended control standards for effective aluminum in zinc liquids are: about 0.16% for galvanized products (GI) and between 0.135% and 0.14% for galvanized iron alloy products (GA).
Twist Rod Nails,Painted Steel Nails,Polisher Vertical Lines,Bamboo Steel Nails
CANGZHOU LINGANG YUANFENG IMPORT & EXPORT TRADING CO.,LTD , https://www.clyfhardware.com